In the research of wheat gluten meat processing, the crispy technology of wheat high-protein foods and wheat gluten protein film forming technology were conquered, and products such as vegetarian fish maw and protein brittleness were developed. Establish a demonstration line for convenience food pilot test. The product has a crisp protein content of >20% and has a crisp and crisp taste. The thickness of the fish belly film is 70%. Protein crisp, vegetarian fish maw and other convenient vegetarian food products have already entered the market for trial sale, and the market feedback is good.
In the research of fortified flour preparation technology, vitamin A microencapsulation research was conducted. The product contained vitamin A microcapsules with an embedding rate of >80%, white powdery particles, and hygroscopicity:
In the comprehensive utilization of wheat starch wastewater, the concentration technology of wheat starch overflow, enzymatic extraction of ultra-fine starch technology, and ultra-low starch low-temperature drying technology were studied. Pure starch composed of ultra-fine particles was prepared. The particle size distribution of starch in the product is approximately: 56% of the diameter of 2-6 microns, 30% of 6-10 microns, 6% of 10-12 microns, 8% of the larger than 12 microns, can meet the thin film Production requirements.
1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff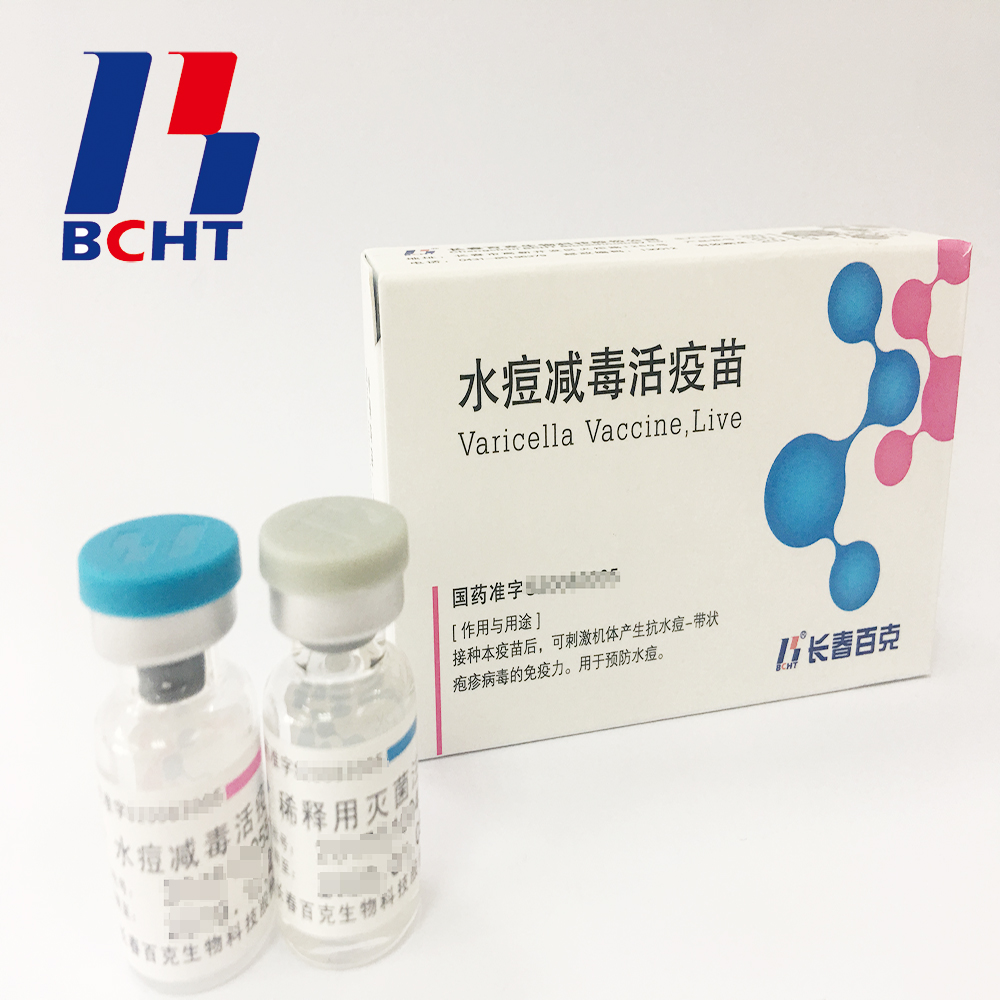 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
Varicella Vaccine(Live)
Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live
Changchun BCHT Biotechnology Co. , https://www.ccbcht.com