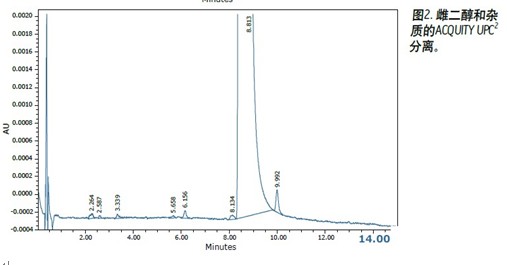
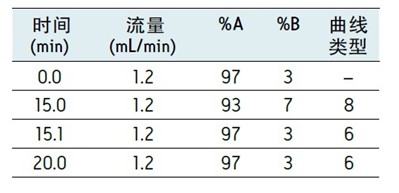
The conditions of the UPC2 method are as follows:
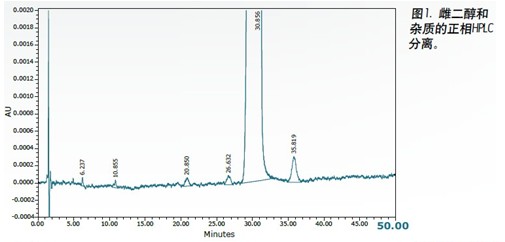
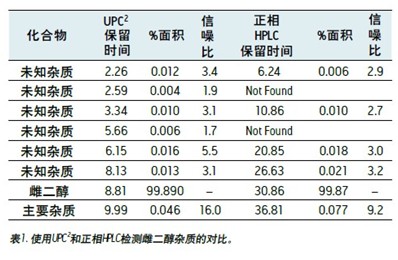
The results obtained by the two test methods are compared in Table 1. Both the normal phase HPLC method and UPC 2 detected at least 5 impurities in an amount of less than 0.1% by area. In both methods, the signal-to-noise ratio of the peak in the range of 0.01% is about 3:1, and the value obtained by the UPC 2 result is slightly higher. The maximum impurity (about 0.05% by area) measured by the UPC 2 method has a signal-to-noise ratio of 16:1 and the normal phase HPLC method measures 9:1. These experimental results clearly show that the ACQUITY UPC 2 system can be successfully used to analyze trace impurities in estradiol. The run time of the UPC 2 method was significantly shorter than the time used for the normal phase HPLC method (20 min vs. 60 min), thereby increasing laboratory productivity. Cost analysis for each run showed that the solvent cost for normal phase HPLC was $5.89, while the cost for each run was only $0.05 with UPC 2 . The mixed chloride waste liquid to be treated by the normal phase HPLC method was 108 Ml 2 , 2,4-trimethylpentane, 9.6 mL of n-butyl chloride and 2.4 mL of methanol. The waste liquid to be treated produced by the UPC 2 method was 0.60 mL each of methanol and isopropanol. The CO 2 used in the separation is discharged through a laboratory exhaust pipe. Using the UPC 2 method, waste disposal costs are reduced by as much as 150 times. 2,2,4-Trimethylpentane, 9.6 mL of n-butyl chloride and 2.4 mL of methanol. The waste liquid to be treated produced by the UPC 2 method was 0.60 mL each of methanol and isopropanol. The CO 2 used in the separation is discharged through a laboratory exhaust pipe. Using the UPC 2 method, waste disposal costs are reduced by as much as 150 times.
About Waters Corporation ()
For more than 50 years, Waters Corporation (NYSE: WAT) has made significant advances in medical services, environmental management, food safety and global water quality monitoring by providing practical and sustainable innovations that have created business for laboratory-related organizations. Advantage.
As a pioneer in a range of separation science, laboratory information management, mass spectrometry and thermal analysis technologies, Waters technology's breakthroughs and laboratory solutions create a lasting platform for customer success.
With a $1.85 billion revenue in 2011, Waters continues to lead customers around the world in exploring science and achieving excellence.
SARS-CoV-2 Neutralizing Antibodies Test (ELISA)
Covid-19 Neutralizing Antibody Test,Covid-19 Neutralizing Antibodies Test,Sars-Cov-2 Neutralizing Antibody Test,Sars-Cov-2 Neutralizing Antibodies Test
Wuxi BioHermes Bio & Medical Technology Co., Ltd. , https://www.biohermesglobal.com